A group from Washington University in St. Louis, School of Medicine, USA, etc. has reported a new mechanism of SARS-CoV-2 infection.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8220945/
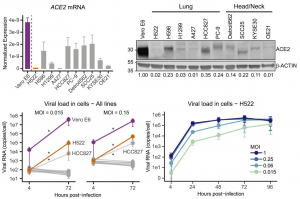
Human lung and head/neck cancer cell lines express varying levels of ACE2 and TMPRSS2. Quite interestingly, H522 human lung adenocarcinoma cells were infected with SARS-CoV-2, despite no evidence of detectable ACE2 and TMPRSS2 expression. Using orthogonal assays, it was confirmed that H522 cells are infected independently from the existence of ACE2, which suggests the utilization of an alternative receptor in a cell line of lung origin. Although recent findings establish Neuropin 1 (NRP1), AXL, and heparan sulfate as mediators of ACE2-dependent SARS-CoV-2 entry, it was found that SARS-CoV-2 infection of H522 cells is independent of Neuropin 1 (NRP1) and AXL but dependent on heparan sulfate.
To decipher the mechanisms of SARS-CoV-2 entry into H522 cells, infection inhibition experiments were performed in the presence of compounds that potentially interfere with SARS-CoV-2 entry, including camostat mesylate (TMPRSS2 inhibitor), E64D (broad spectrum inhibitor of proteases, including endosomal cathepsins: proteases (enzymes that degrade proteins) found in all animals as well as other organisms), bafilomycin A (inhibitor of vATPase), and apilimod (inhibitor of PIKfyve), SGC-AAK1-1 (specific inhibitor of AAK1 kinase 1, which promotes clathrin-mediated endocytosis (CME) through phosphorylation of the AP2M1 subunit of the AP2 complex).
E64D, bafilomycin A, SGC-AAK1-1, and apilimod reduced cell-associated viral RNAs in a dose-dependent manner, whereas camostat mesylate increased viral RNA levels. So, these data support an infection mechanism through CME and endosomal cathepsins in H522 cells.