A group from Copenhagen Center for Glycomics, Department of Cellular and Molecular Medicine, Faculty of Health Sciences, University of Copenhagen, Denmark, etc. has reported about the effects of N-Glycosylation in FcγRIIIa interaction with IgG.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9524020/
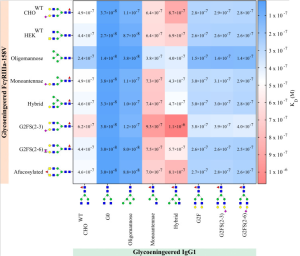
The FcγRIIIa receptor is an activating IgG receptor, mainly expressed on NK cells, macrophages, and monocytes. In this work, the effects of N-glycosylation of FcγRIIa onto affinity between FcγRIIIa and IgG1.
The highest affinity of all FcγRIIIa receptors was observed to afucosylated IgG, both IgG1-G0 and the IgG1-Oligomannose, as is expected.
Interestingly, the N-glycosylation state of the FcγRIIIa had minimal effect on the binding affinity when probed with afucosylated IgG, except for oligomannosylated FcγRIIIa where binding affinity is increased by a factor of two.
The highest KD, i.e., lowest affinity, was seen for IgG1-Hybrid and IgG1-Monoantennae to all FcγRIIIa.
On the other hand, the lowest KD, i.e., highest affinity, was seen for afucosylated IgG1 and oligomannosylated FcγRIIIa.