A group from Department of Protein Engineering, Faculty of Biotechnology, University of Wroclaw, Poland, etc. has reported that the clustering of FGFR triggered by galectins (Gal-1, Gal-s, Gal-7 and Gal-8) binding to those N-glycans can activate the receptor and initiate downstream signaling cascades.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10070233/
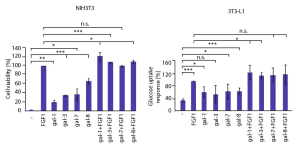
It was demonstrated that galectins modulate FGF/FGFR cellular processes through direct action on the receptor and the cellular consequences of galectin-induced FGFR signaling largely differ from those achieved by the canonical ligand (FGF).
Galectin-1, -3, -7, and -8 were the most effective binders of FGFRs (FGFR1–FGFR4) among human galectins, and FGFR1 clustering promoted by multivalency of galectins was essential for FGFR1 activation and initiation of downstream signaling cascades. Interestingly, the combination of galectin-1, -3, and -8 with FGF1 enhanced the number of viable cells more effectively than each single protein. And further, glucose uptake was enhanced by mixtures of FGF1 with galectin-1 and -3 compared to treatments with each single protein.